Sealing mechanism
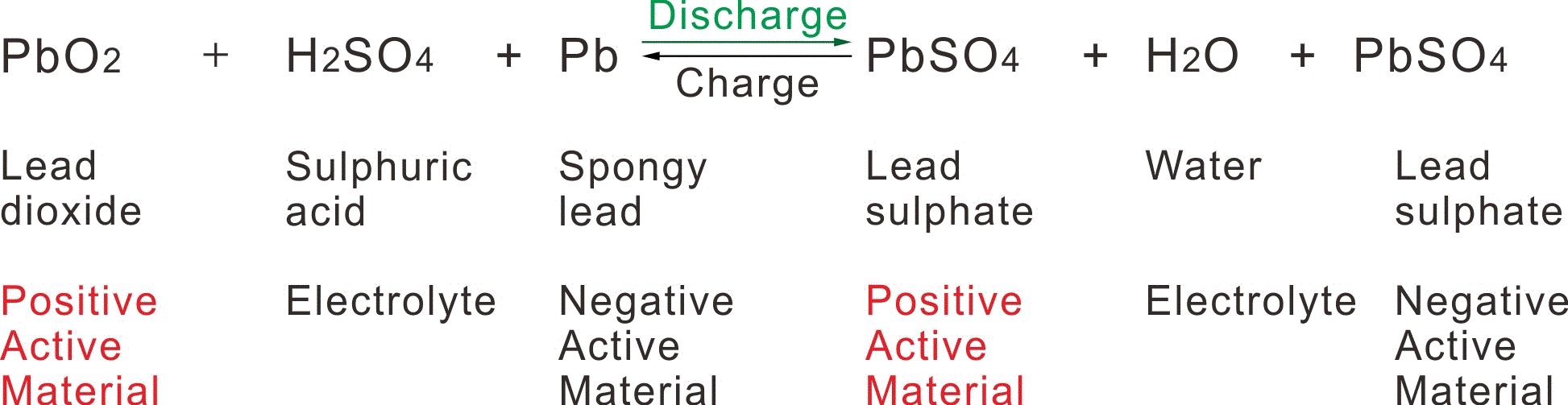
The Chemical reaction taking place in a lead-acid battery is as shown in the following formula :
When battery charging approaches its final stage ,the charging current is consumed solely for electrolytic decomposition of oxygen gas from postitive plates and hydrogen gas from negative plates,the generated gas will escape from the battery cuasing a decrease of the electrolyte,thereby requiring occasional water replenishment.
However,Maxton battery utilize the characteristics of spongy lead,or negative active material,which is very active in moist conditions and reacts very quicky with oxygen,thereby suppressing the decrease of water eliminating the need of water replenishment.
The process of charging from its beginning to the final stage is identical with that of conventional batteries as shown above fomule.
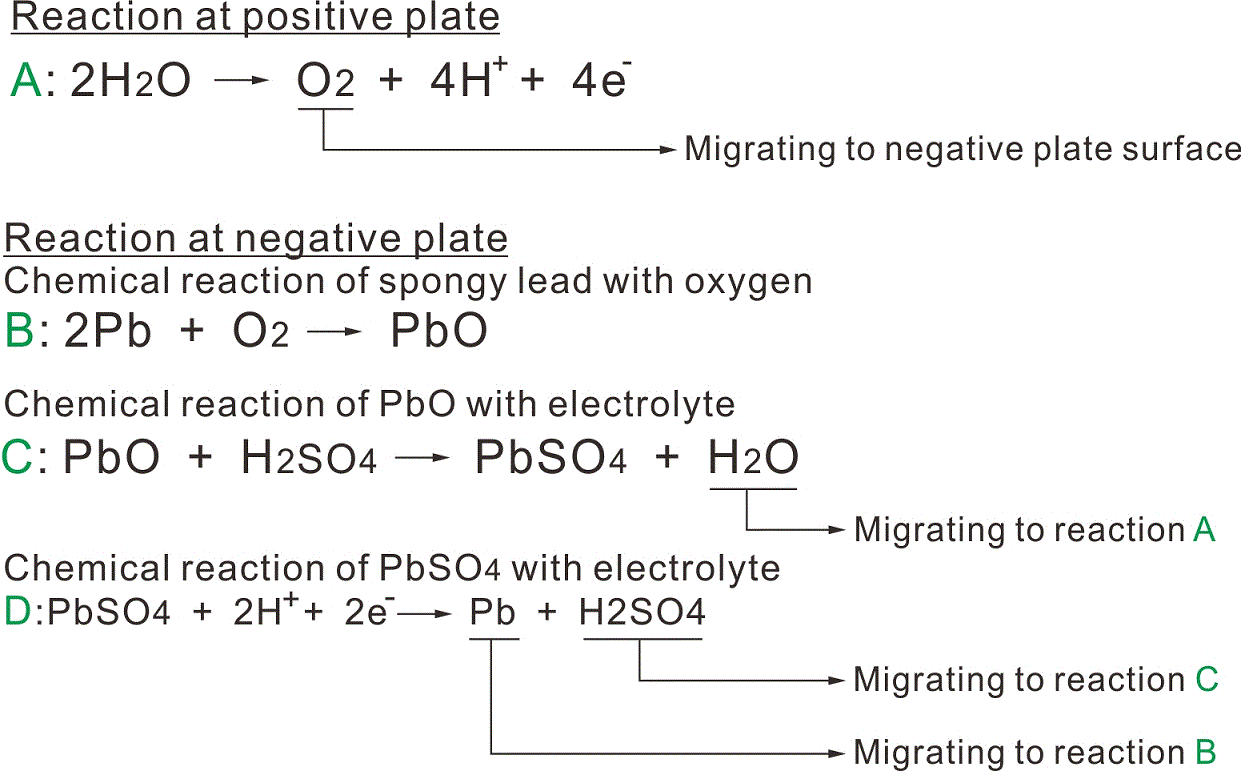
On the second,after the final stage of charging or under overcharge condiiton,the charging energy is consumed for electrolytic decomposition of water,and the positive plates generate oxygen gas,which reacts with the spongy lead in negative plates and the sulphuric acid in electrolyte,turning a part of negative plates into a discharged condition thus suppressing the hydrogen gas generation from nagative plates.
The part of negative plates which had turned to discharged condition through reaction with oxygen gas is the reverted to original spongy lead by subsequent charging.Thus,a negative plate keeps equilibrium between the amount which turns into spongy lead by charging and the amount of spongy lead which turns into lead sulphate through absorbing the gas generated from positive plate,which makes it possible from the battery to be of a sealed type.
The chemical reaction which takes place after the final stage of charging or under overcharge condition is an show in Fig 2.and the reaction formula is described in A,B,C,D.
 Chinese
Chinese